On December 3rd, the Center for Drug Evaluation (CDE) released three guiding principles related to gene therapy and cellular therapy, incorporating organoids into the guiding principles for gene therapy and gene-modified cellular therapeutic products for the first time.
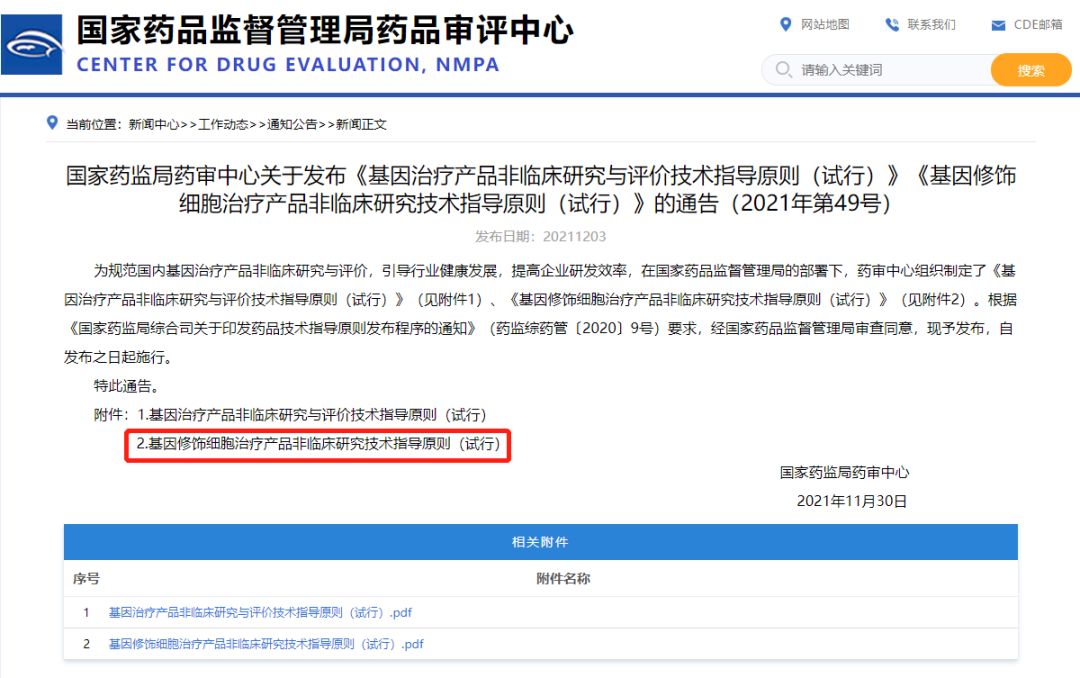
Details are as follows:
① "Technical Guiding Principles for Nonclinical Research and Evaluation of Gene Therapy Products (Trial)" states:
I. For pharmacological studies of gene therapy products, "if no suitable animal models are available to meet the needs of the experiment, corresponding animal models should be developed based on scientific principles, or more sophisticated in vitro test systems or alternative models (such as organoids) should be used for testing."

II. Due to differences in species and immune status, the expression, distribution, and function of gene therapy products in humans may vary significantly in model animals. Alternative products (such as gene-modified model animal cells, tissues, and organoids) can be selected for Proof of Concept (POC) studies.

②"Technical Guiding Principles for Nonclinical Research of Gene-Modified Cell Therapy Products (Trial)" states that:
Cell- and tissue-based models (such as organoids) can be used to provide useful supplementary information for predicting the effectiveness and safety of gene-modified cells in patient populations.

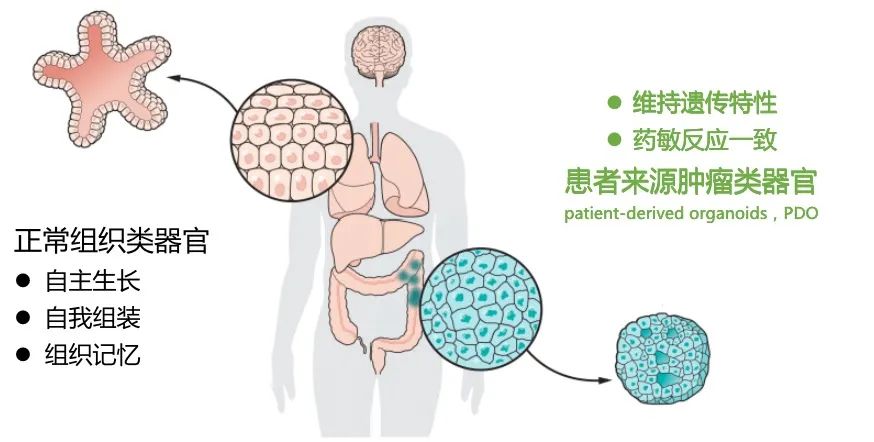
Advantages of organoid models
Organoids refer to tissue-like structures formed through the three-dimensional (3D) culture of adult stem cells or pluripotent stem cells in vitro. Organoids can maximize the simulation of in vivo tissue structure and function and can be stably passaged and cultured for a long period of time.
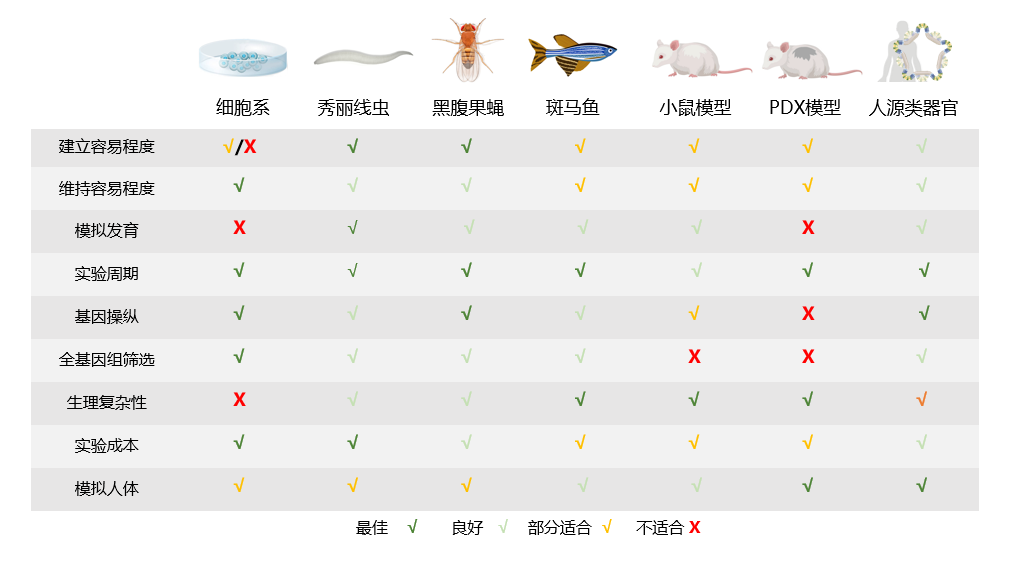
Comparisons among common scientific research models