Organoid culture technology holds promising prospects in regenerative medicine. Organoids are capable of maintaining the critical functions and characteristics of their original tissues, possessing potential for tissue repair. However, the ability of organoids to survive and facilitate tissue repair after transplantation has only been demonstrated in animal studies, and has not yet been proven in humans.
The biliary system transfers bile from the liver to the duodenum. Diseases of the biliary system account for 70% of pediatric cases, but there is a shortage of liver sources for liver transplantation, making therapeutic alternatives urgently needed. The characteristics of the biliary system make it highly suitable for organoid transplantation research. Cholangiocytes can be easily obtained from biliary epithelial cells. Cholangiocytes also share features with other luminal organs, exhibiting distinct transcriptional and functional characteristics along the biliary tree. However, the regenerative capacity of organoids derived from cholangiocytes at different locations within the biliary tree remains unclear.
Recently, a team led by Ludovic Vallier from the Wellcome–MRC Cambridge Stem Cell Institute published an article titled "Cholangiocyte Organoids Can Repair Bile Ducts After Transplantation in the Human Liver" in Science. This article successfully demonstrated biliary regeneration through biliary organoid transplantation.
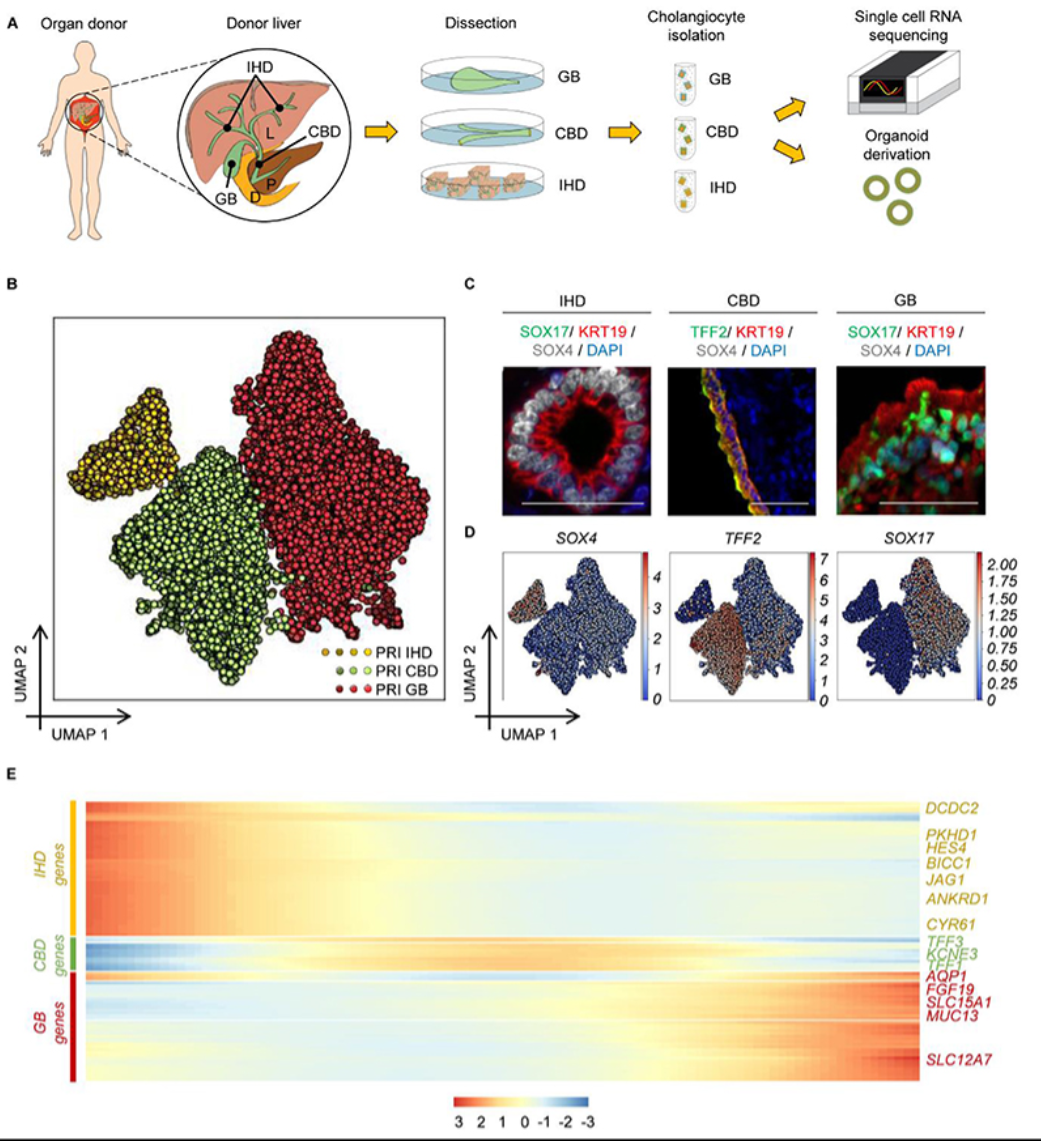
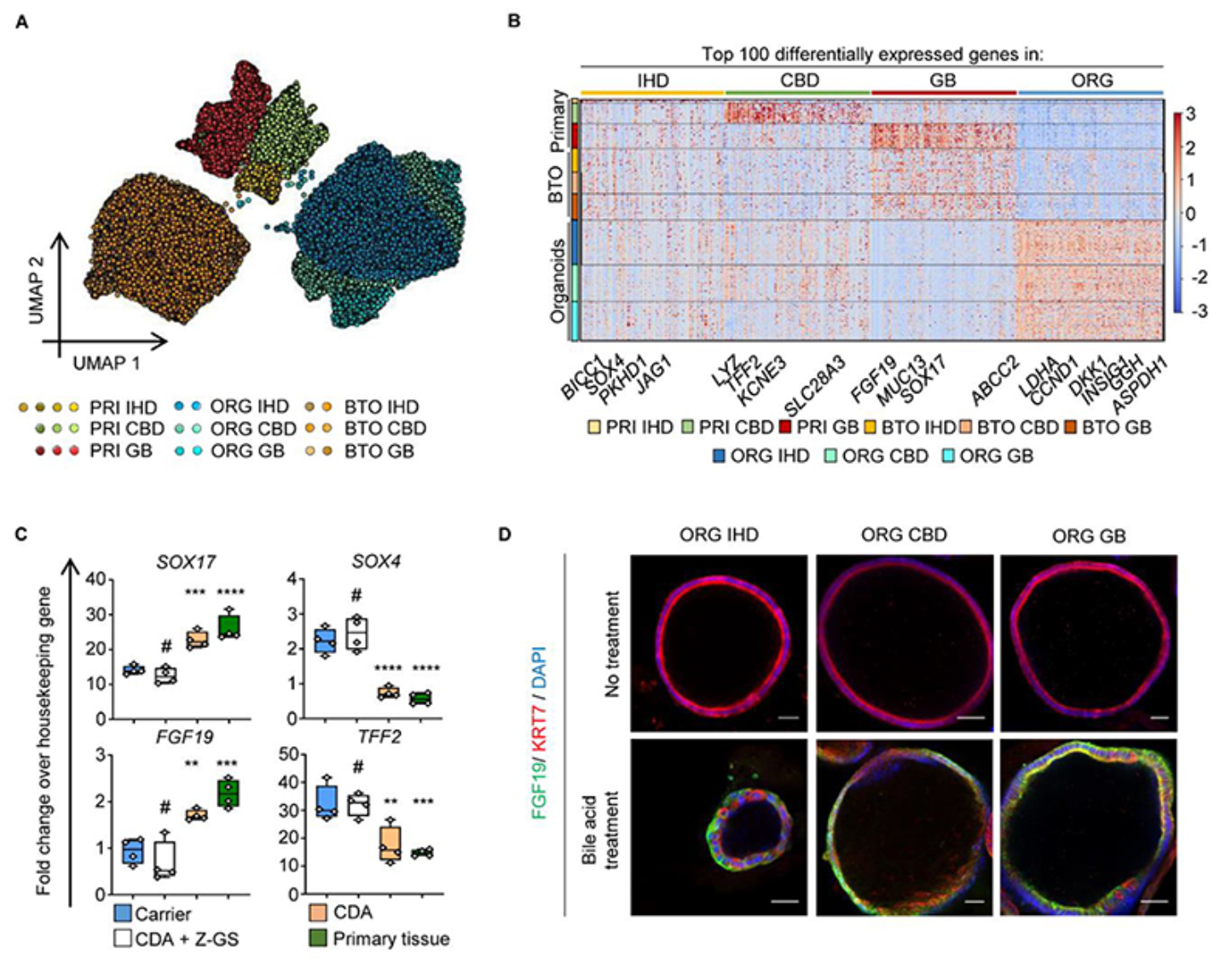
The authors first employed single-cell technology to analyze the transcriptome characteristics of different regions of the human biliary tree, confirming that cells from different regions of the biliary tree exhibit distinct transcriptional profiles. They isolated cholangiocytes from intrahepatic bile ducts, common bile ducts, and gallbladders. All three groups of cells shared a core transcriptional profile, but each region's cholangiocytes had unique gene expression signatures. These gene expression signatures corresponded to their functions and reflected the cells' adaptation to their microenvironments. However, when single-cell transcriptome analysis was applied to biliary organoids, the differences in transcriptional profiles between cells disappeared, and the profiles overlapped, indicating that the biliary cells grown in vitro were no longer influenced by their original regions but instead adapted to the changes in the new environment. To investigate the plasticity of biliary organoids, the authors cultured the cells in different microenvironments and found that the regional specificity of the cells could be restored through induction by the external environment.
Next, the authors used a mouse model of biliary injury to explore whether repair of another region of the biliary tree could be achieved through cellular plasticity, thereby advancing organoid-based organ repair techniques. Biliary lesions were induced by MDA in immunodeficient mice. Control mice died within three weeks, while mice receiving biliary organoids survived for up to three months. Organoids were transplanted into intrahepatic bile ducts of varying sizes, accounting for about 25%-55% of the regenerated bile ducts. Examination and testing revealed no expression of other liver lineage markers, indicating that the plasticity of biliary organoids was limited to the biliary lineage. The authors repeated the experiment using mesenchymal stem cells, but this did not improve the survival of the mice and showed no significant difference compared to the control group, suggesting that regenerative therapy for biliary diseases requires the use of organoids derived from biliary cells.
Comparison between Organoid Transplantation Group and Control Group
The mouse transplantation experiments were effective, but they could not predict the outcomes when applied to humans. To investigate this, the authors used an ex vivo normothermic perfusion (NMP) transplantation model. The NMP system is designed to improve organ preservation and reduce ischemia-reperfusion injury, maintaining organs in near-physiological conditions for up to 100 hours. By labeling biliary organoids with RFP, the authors examined the changes after organoid transplantation. In the injected biliary tree, the graft regeneration rate was approximately 40-85%, and the grafts expressed key biliary markers. This was also the first successful transplantation of biliary organoids in human perfused organs, facilitating regeneration of the biliary system.
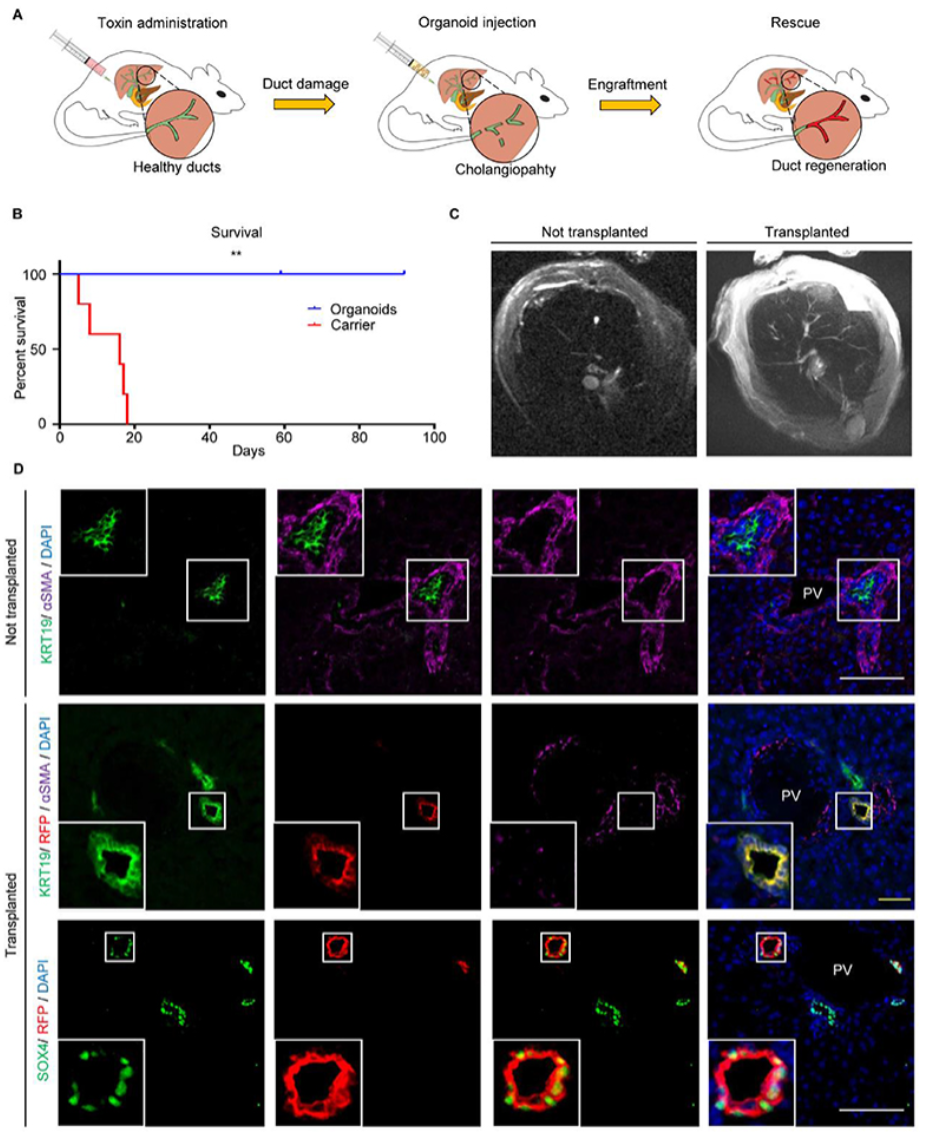
Diagram of Biliary Organoid Transplantation in an Ex Vivo Tissue Perfusion Model
Simone N. T. Kurial and Holger Willenbring from the University of California, San Francisco, have published a perspective article titled "Emerging Cell Therapy for Biliary Diseases" on this research.
They point out that cell therapy has the potential to replace liver transplantation. Despite the numerous challenges, an increasing number of studies support the feasibility of cell therapy for intrahepatic biliary diseases. More research is needed to address the issue of selecting the appropriate cell type for implantation, improve implantation efficiency, and fill the gaps in research on the safety of cell therapy. This study represents the first successful application of biliary organoid transplantation in regenerative medicine, providing a prototype for the use of organoids in humans and accelerating the clinical application of cell therapy.