With the development of life sciences, humans' desire to better understand themselves and overcome various diseases can no longer be satisfied solely by animal models. Replicating and reconstructing human organs has become one of the directions scientists are attempting to pursue. Thus, organoids were born. Organoids are three-dimensional cellular complexes formed through the induced differentiation of stem cells or organ progenitor cells using 3D culture techniques in vitro, which resemble the target organ or tissue both structurally and functionally. They possess stable phenotypes and genetic characteristics and can be cultured long-term in vitro. During their formation, they recreate two events of organogenesis in vivo: the adhesion-based sorting and aggregation of similar cells and the spatial specification of cell lineages [1]. Compared to traditional 2D cell culture models, 3D-cultured organoids contain multiple cell types, transcending simple physical cell-cell contacts to form tighter intercellular biological communication, where cells influence, induce, and provide feedback to each other [2]. They collaborate to develop and form functional mini-organs or tissues, which can better simulate the ontogeny and physiological and pathological states of organ tissues, thus holding broad application prospects in basic research and clinical diagnosis and treatment.
However, traditional 3D culture techniques that rely on sequentially adding growth factors to culture mammalian stem cells to form organoids still have limitations. For example, they lack precise control over the organoids and their local environments. Additionally, this method fails to adequately replicate the complex and dynamic microenvironments during organ development, which are crucial factors for organ formation [3]. This makes it difficult to obtain more complete organoids that resemble in vivo organ development [4].
To address the limitations of traditional culture techniques, experts in stem cell and developmental biology have collaborated with physicists and engineers to develop more advanced in vitro techniques for organoid research. Currently, at the forefront of this research is the integration of organ-on-a-chip technology with organoids, resulting in "organoid-on-a-chip" technology. Recently, Sunghee Estelle Park, Andrei Georgescu, and Dongeun Huh, professors from the University of Pennsylvania, co-authored a review titled "Organoids-on-a-chip" for Science, reviewing recent research that applies organ-on-a-chip technology to organoids, elucidating in detail how organ-on-a-chip technology helps address significant technical challenges in organoid research, and presenting forward-looking insights for the application of organoid-on-a-chip technology.

What is Organ-on-a-chip?
In a broad sense, organ-on-a-chip is a miniature cell culture device that simulates the unit functions of human organs in vitro, with microfluidic chip technology as its core. Generally speaking, the establishment of an organ-on-a-chip system is guided by design principles based on simplified analysis of the target organ. Firstly, it is crucial to understand the anatomical structure of the target organ and reduce it to the essential components necessary for physiological function. These functional units are further used to identify key features such as different cell types, structural organizations, and organ-specific biochemical and physiological microenvironments. Then, a cell culture device is designed to replicate these features and manufactured through microfabrication techniques such as soft lithography (Figure 1). By integrating human live cells with physiologically relevant microenvironments, organ-on-a-chip can simulate organ-level functions that are important for physiological homeostasis and complex disease processes. This makes them promising for supplementing and reducing animal experimentation in preclinical applications of drugs, medical devices, and biomaterials, and also provides a favorable in vitro platform for screening the side effects of chemicals, environmental materials, and consumer products.
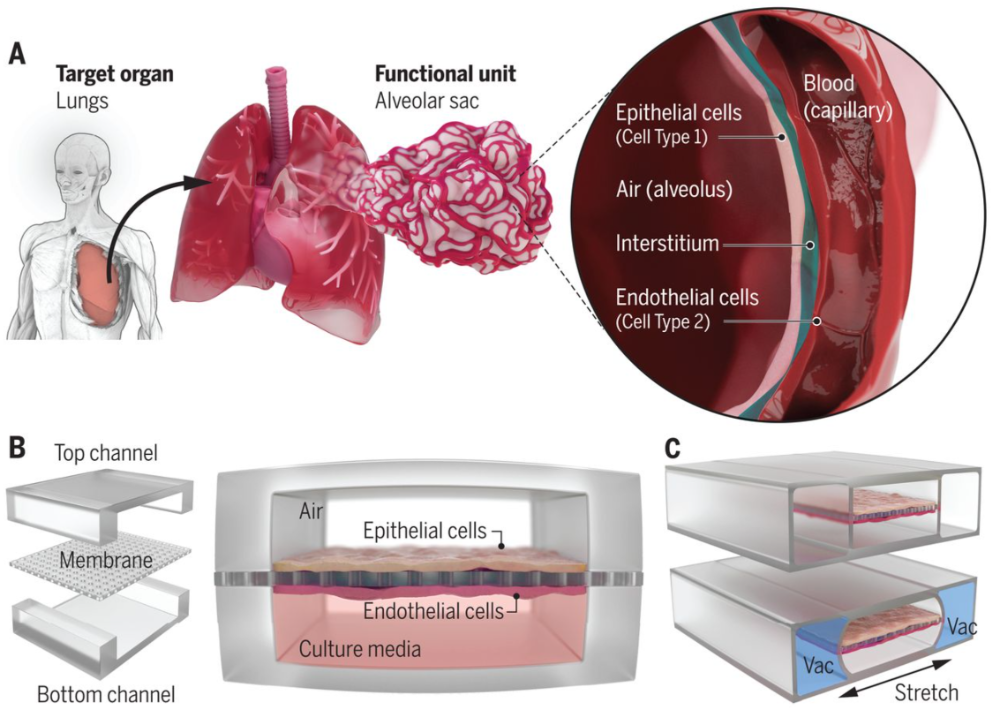
Figure 1: Design Principles of Lung Organ-on-a-Chip
What can organ-on-a-chip technology bring to organoids? The authors of this article explain it from three major aspects.
I. Control Over the Microenvironment of Organoids
The development of organoids requires the activation of relevant signaling pathways at the right time and in a controllable manner to induce cell fate differentiation and physical separation into different cell types, and to guide self-organization. Traditional organoid cultures require the addition of morphogens at specific times to form a biochemical gradient microenvironment for stem cells along with soluble factors secreted by cells. However, this gradient is difficult to control and has a low success rate. Increasingly, studies have found that combining organ-on-a-chip technology with organoid cultures can solve this problem. A representative example is the application of a microfluidic system to establish an in vitro model of neural tube development (Figure 2A). This microdevice simulates the Sonic Hedgehog (Shh) and Bone Morphogenetic Protein (BMP) signaling pathways, successfully inducing the formation of the neural tube during spinal cord development. Similar methods have also successfully generated chemical gradient microenvironments, allowing human brain organoid-on-a-chip to be used to study the adverse effects of nicotine on cortical development, and so on.
Furthermore, as organoids grow larger, their reliance on passive diffusion for nutrient and oxygen acquisition and metabolite removal becomes insufficient to meet their increasing metabolite demands, ultimately leading to failed growth and maturation. Organ-on-a-chip models simulating perfused blood vessels have proven to solve this problem. A tumor organoid-on-a-chip model contains three interconnected microfluidic devices (Figure 2B), supporting the self-assembly of endothelial cells into 3D perfused blood vessels and the growth of organoid structures from breast cancer patients. Using this device, paclitaxel perfused through the blood vessels can inhibit tumor growth, and it can also be used to screen personalized drugs for patients.
Additionally, we know that embryonic development involves various types of mechanical forces, ranging from traction forces generated by single cells to fluid shear stress and solid mechanical forces caused by the mechanical activity of integrated cell populations. These forces, along with soluble morphogens and extracellular matrix signals, regulate organ development and maturation. Traditional organoid models lack biomechanical control, leading to incomplete development. The application of microengineering devices can generate mechanical forces similar to those in vivo for organoids, such as a stomach-on-a-chip model where stomach organoids derived from human pluripotent stem cells are cultured on artificial basement membranes. The hollow structure is paired with a micropipette to allow liquid to flow into the lumen, simulating the luminal flow and rhythmic contractions of the stomach in vivo (Figure 2C).
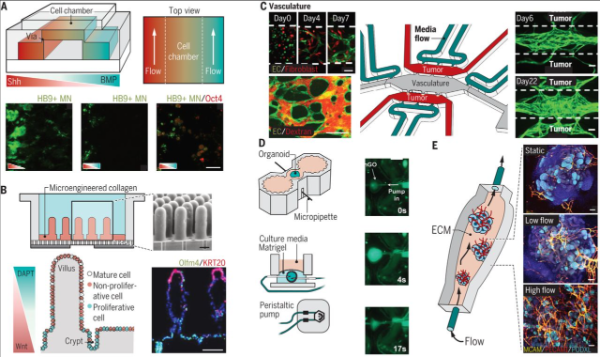

Figure 2: Organoid-on-a-Chip for Controlling the Microenvironment
II. Establishing Interactions Between Tissues and Multiple Organs
From a systemic perspective, the complexity of the human body arises from the dynamic interactions between different tissues and their internal components. Therefore, simulating these interactions is crucial for modeling complete and complex physiological systems in vitro. Organ-on-a-chip provides a more controllable and advantageous design platform for co-culturing different cell and tissue types within the organoid system. For instance, a multi-chamber microdevice establishing a vascular-liver organoid-on-a-chip enhances the interaction between liver tissue and blood vessels in vitro, thereby increasing the expression of liver-specific markers and enhancing liver function in vitro. Additionally, studies have successfully co-cultured different types of organoids using in vitro microplatforms, including liver, intestine, and stomach organoids derived from stem cells. Such multi-organ systems will aid in predicting the application patterns of biopharmaceuticals in preclinical settings.
III. Reducing Variability
Unlike in vivo organ formation, the development of organoids exhibits considerable variability in size, structural organization, function, and gene expression. Organ-on-a-chip technology offers a method for organoids to produce patterns similar to the tightly regulated organ formation in vivo, thereby reducing randomness and variability. Specifically, first, the precision and reproducibility of mechanical automation in microdevices reduce the variability introduced by manual inconsistencies during experimental procedures (such as organoid culture). Second, microengineering devices increase the density of organoid cultures, enabling high-throughput processing and analysis. Finally, by incorporating biosensing elements into the culture platform, organoids can be continuously screened. For example, a study designed a multi-organ chip device integrated with label-free biosensors for long-term monitoring of heart and liver organoids and primary liver spheroids, enabling simultaneous monitoring of up to eight different targets with high sensitivity and over a wide dynamic range (Figure 3).

Figure 3: Multi-Organ Platform with Integrated Sensors Facilitates In Situ Monitoring of Organoids
In summary, the organoid-on-a-chip, resulting from the combination of two technologies, complements each other's strengths and weaknesses, making it a more useful and predictive preclinical experimental model. It can be widely applied in the development process of traditional or new drugs. By using tissue samples from patients as organoids, the organoid-on-a-chip can also create specific disease models, enabling precision medicine. Additionally, the organoid-on-a-chip can facilitate the development of regenerative medicine by screening optimized organoids under in vitro conditions through high-throughput analysis.
Despite the promising application prospects of the organoid-on-a-chip, its limitations still deserve our attention. For example, since the design and establishment of organoid-on-a-chip models are predetermined, there are still challenges in addressing the dynamic changes in structure, environment, and function during organ formation. Therefore, how to better integrate organ-on-a-chip technology with organoids remains a scientific issue with great development potential. Scientists around the world need to actively communicate and work together to create a better era of organoids!
Link for the original text:
https://science.sciencemag.org/content/364/6444/960
References
1. LancasterMA, Knoblich JA. Organogenesis in a dish: modeling development and diseaseusing organoid technologies.Science2014;345 (6194) :1247125.
2. Sasai Y.Next-generation regenerative medicine: organogenesis from stem cells in 3Dculture. Cell StemCell 2013;12 (5) :520-30.
3. McCauley HA,Wells JM. Pluripotent stem cell-derived organoids: using principles ofdevelopmental biology to grow human tissues in a dish, Development 144, 958–962(2017).
4. X. Yin etal. Engineering Stem Cell Organoids. Cell Stem Cell 18, 25–38 (2016).









