The two recent articles published in the journal Nature showcase scientists' efforts in elucidating the pathogenic mechanisms of Apc mutant cells in intestinal tumors with the help of organoids, and propose effective therapeutic measures.
Mutations in the tumor suppressor gene Apc and its competitive inhibition of the WNT signaling pathway are the main causes of intestinal cell carcinogenesis. Apc is a gene that regulates cell proliferation, migration, adhesion, and chromosomal stability. The Apc protein produced by this gene maintains cellular balance by degrading related proteins through complex formation. On the other hand, the WNT signaling pathway is a complex network composed of various signaling proteins that is activated by the binding of the ligand protein WNT to receptors. It is involved in the regulation of embryonic development, cancer, and various physiological processes. These two factors play crucial roles in the development of intestinal tumors. However, the detailed pathogenic mechanisms remain unclear, and effective therapeutic targets have not been identified. An article published in the journal Current Biology in 2016 by Eugenia Piddini et al. demonstrated that Apc mutant cells have a competitive advantage over wild-type cells in Drosophila. This paper showed that Apc mutant clones in the Drosophila midgut have been proven to actively induce apoptosis in surrounding tissues (Figure 1).
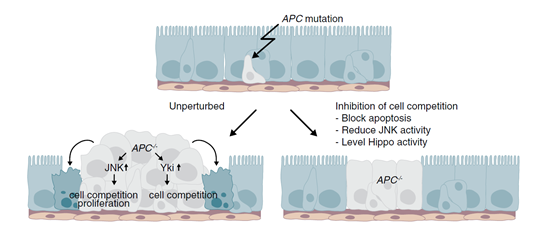
However, using Drosophila as a model cannot effectively demonstrate the process by which Apc mutations function in humans. On June 2nd of this year, Sanne M. van Neerven, Nina E. de Groot, and their colleagues published a paper titled "Apc-mutant cells act as supercompetitors in intestinal tumour initiation" in the journal Nature. They utilized intestinal organoids as a model to effectively simulate the carcinogenesis process in human organs, providing a more detailed explanation of how Apc mutant genes exert their competitive advantage to inhibit the normal function of intestinal stem cells. This study offers the potential for identifying effective drug targets for intestinal cancer.

In the niche of intestinal cells, a delicate balance is maintained between WNT agonists and antagonists, which is crucial for maintaining the function of intestinal stem cells and adapting to the self-renewal of the intestinal lining. However, mutated tumor suppressor Apc disrupts this balance. Studies have shown that Apc mutant cells have a competitive advantage over intestinal stem cells, leading to the unlimited activation of intestinal stem cells and inducing tumorigenesis. However, it remains unclear whether the carcinogenic process only involves the intrinsic characteristics of Apc mutant cells, or whether these mutant cells actively eliminate surrounding normal cells.
To further explore how Apc mutant cells exert their competitive advantage, the authors established a coculture system of wild-type and Apc-/- organoids and conducted research around the cultivated organoids. There were three coculture systems: 1) coculture of two wild-type intestinal organoids; 2) coculture of wild-type/Apc-/- organoids; and 3) culture of wild-type organoids in medium conditioned by Apc-/- cell secretions. By observing the growth status of the organoids, the researchers found that Apc mutant organoids and their secreted factors actively inhibited the cell expansion and proliferation of wild-type organoids (Figure 2).

Figure 2. Apc mutant cells actively inhibit the growth of wild-type organoids.
Subsequent transcriptome analysis revealed that wild-type organoid differentiated cells (MUC2 goblet cells) were more abundant than stem cells (Lgr5+ cells) in the medium conditioned by Apc-/- cell secretions (Figure 2). This suggests that Apc mutant cells inhibit the growth and clonogenic ability of wild-type organoids by inhibiting proliferation and promoting differentiation.
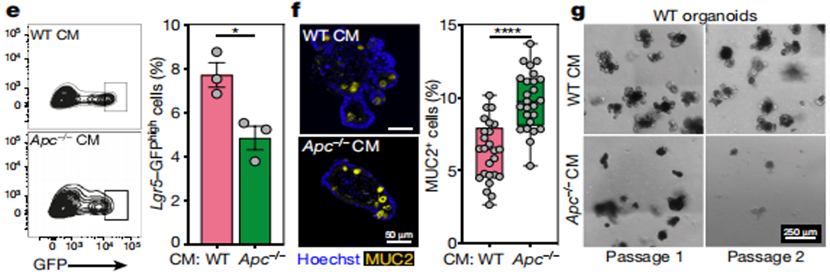
Figure 3. Apc Mutation Promotes Differentiation of Wild-Type Cells
Transcriptome analysis also showed upregulation of WNT antagonists, with factors such as Notum being particularly prominent. This indicates that Apc mutations also inhibit the growth of wild-type organoids by secreting WNT antagonists.
The research results suggest that sustained WNT pathway signaling leads to the activation of an effective negative feedback loop, including the upregulation of WNT antagonists, which may regulate WNT levels in the physiological environment. The addition of WNT activators such as GSK3-β inhibitors and lithium chloride, which stimulate the WNT pathway and inhibit antagonists like Notum, offers new possibilities for the prevention and treatment of intestinal cancer (Figure 4).

Figure 4. Lithium Chloride Neutralizes Apc Inhibitory Capacity, Reducing Tumor Formation
Meanwhile, another article titled "NOTUM from Apc-mutant cells biases clonal competition to initiate cancer," published in the Nature journal by Dustin J. Flanagan, Nalle Pentinmikko, and others, also utilized organoids to study tumorigenesis caused by Apc mutations. Unlike the previous study, this research provided detailed insights into the role of NOTUM in the process.
 By utilizing mouse models for gene upregulation studies, transcriptome analysis, and immunofluorescence staining, NOTUM emerged as the most prominent factor in the tumorigenesis induced by Apc mutant cells. However, even though mice are excellent experimental animals, they cannot perfectly recapitulate human physiological processes. To further investigate, the authors chose to culture small intestinal organoids in conditioned media. In Apc-/- conditioned media, consistent with previous studies, the growth of wild-type organoids was inhibited, with a more pronounced decrease in their number over time. Additionally, stem cell markers decreased while differentiation markers increased. The number of wild-type organoids began to recover after the addition of a NOTUM inhibitor (Figure 5).
By utilizing mouse models for gene upregulation studies, transcriptome analysis, and immunofluorescence staining, NOTUM emerged as the most prominent factor in the tumorigenesis induced by Apc mutant cells. However, even though mice are excellent experimental animals, they cannot perfectly recapitulate human physiological processes. To further investigate, the authors chose to culture small intestinal organoids in conditioned media. In Apc-/- conditioned media, consistent with previous studies, the growth of wild-type organoids was inhibited, with a more pronounced decrease in their number over time. Additionally, stem cell markers decreased while differentiation markers increased. The number of wild-type organoids began to recover after the addition of a NOTUM inhibitor (Figure 5).

Figure 5. Apc Mutation Inhibits Wild-Type Organoid Growth through Secretion of Notum
In contrast, wild-type organoids exhibited similar growth dynamics in Apc-/-/Notum-/- conditioned media as they did in regular media, and this growth dynamic was reversed upon the re-addition of Notum.
Subsequently, the authors used β-catenin staining to assess the growth status of Apc mutant individuals in mouse models. The results indicated that Notum drives the expansion and fixation of Apc mutant clones by directly inhibiting WNT signaling and promoting the differentiation of adjacent stem cells while inhibiting their proliferation. Therefore, Notum can serve as a therapeutic target for intestinal tumors caused by Apc mutations, and disease treatment can be achieved through the use of Notum inhibitors (such as NOTUMi) (Figure 6).

Figure 6. Inhibition of Notum Reduces the Impact of Apc Mutant Cells and Tumor Spread
Figure 6. Inhibition of Notum Reduces the Impact of Apc Mutant Cells and Tumor Spread
The results presented in these two studies are exciting. Research on intestinal tumors hit a bottleneck after the discovery of Apc mutant cells and the WNT signaling pathway. However, scientists have now made breakthroughs using organoids as models. By focusing on cell-cell interactions and the microenvironment, they have revealed the impact of Apc mutant cells on the downstream WNT pathway and identified new therapeutic targets for tumors. Compared to biological models such as experimental animals, organoids can perfectly recapitulate human organs and their niches, which will accelerate the translation of experimental results into medical treatments. The future of biomedical science belongs to organoids, and with the continuous development of organoid technology, once incurable diseases will become less daunting!
References:
1.van Neerven, S.M., de Groot, N.E., Nijman, L.E. et al. Apc-mutant cells act as supercompetitors in intestinal tumour initiation. Nature 594, 436–441 (2021).
2.Flanagan, D.J., Pentinmikko, N., Luopajärvi, K. et al. NOTUM from Apc-mutant cells biases clonal competition to initiate cancer. Nature 594, 430–435 (2021).











