In 2019, the emergence of the novel coronavirus SARS-CoV-2 (Severe Acute Respiratory Syndrome-Coronavirus-2) led to a rapid and ongoing global pandemic. The related disease, also known as Coronavirus Disease 2019 (COVID-19), ranges from asymptomatic presentations to severe respiratory syndromes and has resulted in over 5.88 million deaths to date.
COVID-19 primarily affects the lungs, with impairment of smell and taste being the most prominent signs. However, as the disease progresses and investigations deepen, substantial evidence has emerged showing that other organs are also affected by SARS-CoV-2, such as the occurrence of acute kidney injury (AKI).
The incidence of AKI has contributed to the increased morbidity and mortality associated with COVID-19. However, it is currently unclear whether SARS-CoV-2 infection directly targets the kidneys and damages related cells. AKI may be a complication of acute respiratory distress syndrome in COVID-19 patients or it may be caused by direct infection with the virus.
In February 2022, a team led by Jitske Jansen from RWTH Aachen University in Germany published an article titled "SARS-CoV-2 infects the human kidney and drives fibrosis in kidney organoids" in Cell Stem Cell. They used kidney organoids cultivated from human induced pluripotent stem cells (hiPSCs) to visually study the impact of SARS-CoV-2 on the human kidney.

Since the onset of the COVID-19 pandemic, studies have shown that SARS-CoV-2 infection is associated with certain specific proteins and ligands, such as ACE2 and TMPRSS2. The authors conducted staining and comparison experiments on kidney samples from patients and found that the SARS-CoV-2 nucleocapsid protein was present in the cytoplasm of proximal tubule epithelial cells expressing ACE2. Additionally, kidney injury molecule 1 (KIM1) and the occurrence of renal fibrosis were also observed in the staining results. (Figure 1)

Figure 1. Staining Results of Kidney Samples from COVID-19 Patients
Subsequent analyses by the authors, including RNA sequencing, gene set enrichment analysis, inference of activity using pathway response genes, CellPhoneDB, and CrossTalkeR, detected increased expression of SARS-CoV-2 infection-related genes PLCG2 and AFDN, as well as an increase in the expression of collagen, ECM glycoproteins, and proteoglycans in fibroblasts. Furthermore, proximal tubular cells, podocytes, and fibroblasts, as well as the interactions between these cells, were more active compared to normal cells. These results strongly indicate direct infection of the kidney by the SARS-CoV-2 virus and an upregulation of fibrosis-driving pathways.
Based on these experimental results, the authors conducted virus infection tests using kidney organoids cultured from human induced pluripotent stem cells (hiPSCs) to study the most direct effects of the virus on the kidney, avoiding interference from the human body's systems and obtaining the most accurate data. The organoids were constructed by regulating the addition time of the WNT activator CHIR99021 during cell culture to obtain different cell types, which were then mixed in a certain proportion to obtain the morphogenetic factors necessary for segmented pattern growth. Finally, the desired organoids were obtained in a 3D culture environment and subjected to virus infection. Immunostaining verified the presence of nephron-like structures in the organoids, and the expression of viral RNA confirmed successful infection. (Figure 2)
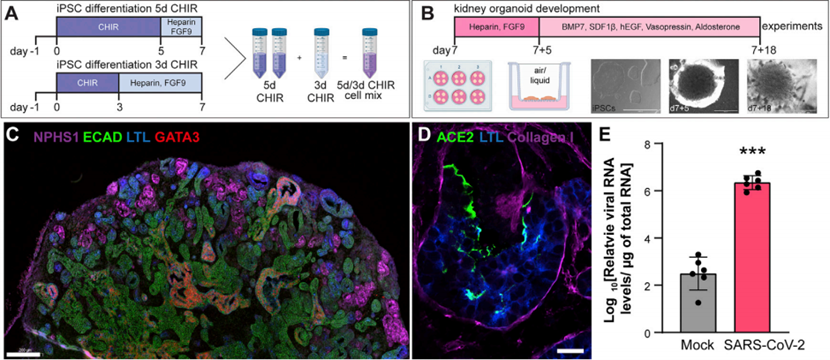
Figure 2. Cultivation and Verification of Kidney Organoids
Subsequently, immunofluorescence staining of various tissue samples confirmed the staining of the viral nucleocapsid protein in podocytes, proximal tubular epithelial cells, and stromal cells. Furthermore, transmission electron microscopy was successfully utilized to identify the ultrastructural features of viral particles in the mesenchymal cells that were positive for SARS-CoV-2 nucleocapsid protein fluorescence. Additionally, immunostaining combined with Masson staining revealed an increase in collagenase I and the degree of organoid fibrosis, indicating the potential for virus infection to induce renal fibrosis. (Figure 3)
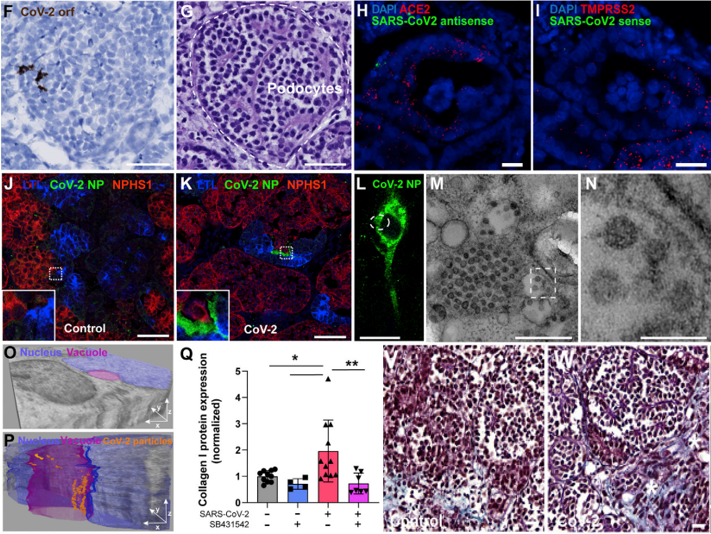
Figure 3. Tissue Staining After Virus Infection
The authors once again performed RNA sequencing on kidney organoids to investigate the mode of viral infection. The results showed that the cell type distribution between the control group and the SARS-CoV-2-infected kidney organoid samples was generally similar. However, compared to the control group, the infected kidney organoids exhibited an increase in neuronal cells and podocytes, along with a decrease in muscle cells. Additionally, similar to previous studies, the authors found upregulated expression of PLCG2 and AFDN in almost all cell populations following SARS-CoV-2 infection. Moreover, SARS-CoV-2 gene expression was detected in 4% to 25% of proximal tubular cells and 1.4% to 18% of podocytes. These results indicate that proximal tubular epithelial cells and podocytes, two important human renal cell types, may be the primary sites of infection for SARS-CoV-2. (Figure 4)
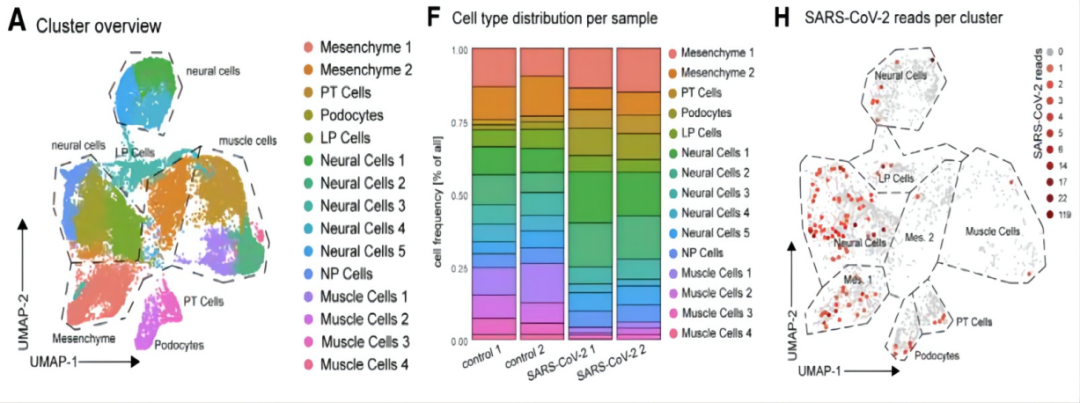
Figure 4. RNA Sequencing of SARS-CoV-2-Infected Kidney Organoids
Next, the authors validated the fibrotic damage observed in patient kidneys. KEGG pathway analysis revealed upregulation of the TGFb, PI3K/Akt, MAPK, and WNT signaling pathways in proximal tubular cells and mesenchymal cells. These pathways play crucial roles in renal fibrosis and their upregulation, which coincides with that of the COVID-19 virus, suggests that the changes are induced by viral infection. PROGENy analysis also demonstrated increased activity of fibrosis-related factors such as MAPK, NFkB, TNFa, WNT, and TGFb. DoRothEA analysis further identified increased activity of MYC, E2F4, serum response factor (SRF), JUN, and SP1 in proximal tubular cells, podocytes, and mesenchymal cells. Finally, CellPhoneDB and CrossTalkeR analyses, focusing on intercellular interactions, revealed that interactions between proximal tubular cells and mesenchymal cell populations, as well as between podocytes and endothelial cell progenitor populations, promote renal cell fibrosis. In summary, SARS-CoV-2 infection induces cellular damage and dedifferentiation in kidney organoids and activates profibrotic signaling. This explains how the novel coronavirus disrupts renal cells and triggers comorbidities such as acute kidney injury. (Figure 5)
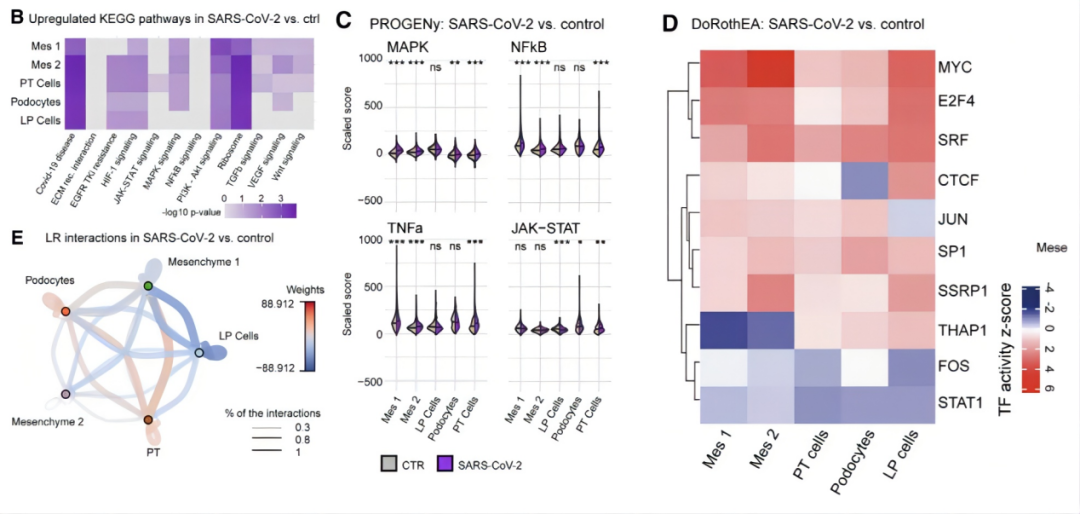
Figure 5. Upregulation of Fibrotic Factors and Pathways in SARS-CoV-2-Infected Kidney Organoids
The novel coronavirus SARS-CoV-2 has continued to exert a profound impact on society and the economy globally since its emergence, severely disrupting people's lives and national development. Consequently, countless scholars have devoted themselves to research in order to solve this worldwide challenge as soon as possible. However, relying solely on clinical data or single-cell experiments is insufficient for effectively understanding the pathogenesis or mechanism of action of the novel coronavirus. Clinical data cannot avoid the influence of the whole on the part, while single-cell experiments cannot effectively reveal the damage caused by the virus to organs.
Therefore, the organoids used in this study not only effectively avoid the influence of the human body's system on a single organ but also effectively simulate the structural and functional characteristics of the kidney, enabling experimental data to accurately reflect the most direct impact of the virus on the human body. Although organoids lack some functions of immune organs, their results indicate that viral infection is at least partially independent of the systemic effects of the disease or intensive care treatment. Thus, viral infection may directly lead to acute injury and fibrotic remodeling of the kidney, resulting in decreased renal function and the development of complications. This article reveals the impact of the novel coronavirus on the kidney through the use of organoids, providing new insights into the study of the virus and the prevention of its complications.









