
To promote the development of the stem cell and regenerative medicine in China, facilitate in-depth collaboration among stem cell researchers, and explore key scientific questions in the field, the 11th Annual Meeting of CSSCR will be held in Guangzhou from December 12 to 15, 2021.
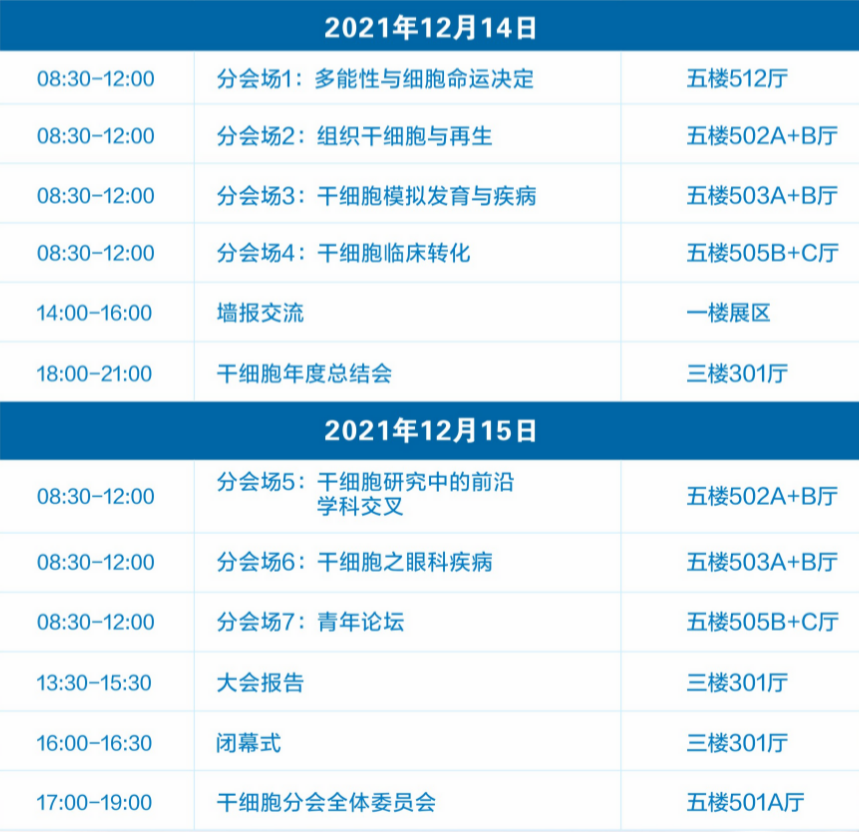
The theme of this year's conference is "Stem Cell Research and Translation: Looking to Southern China" and it will focus on six key topics: pluripotency and cell fate determination; tissue stem cells and regeneration; stem cells simulating development and disease; clinical translation of stem cells; interdisciplinary frontiers in stem cell research; and stem cells in ophthalmic diseases.
On December 14, bioGenous’ Chief Scientist, Professor ZHAO Bing from Fudan University, has been invited to deliver a presentation titled "Organoid Models for Infectious Diseases and Developmental Defects" in the "Stem Cells Simulating Development and Disease" session.

bioGenous Booth: No. 51
A warm "neighborhood vibe" filled the atmosphere

Exhibition Hall Layout
Exhibited Products: "Organoid Culture, Just Right."
Standardized Organoid Culture Kits
Whole-Process Organoid Kits
High-Activity Organoid Culture Growth Factors

*Part pf the product diagram
Visitors to the exhibition can enjoy exquisite gifts. We warmly welcome experts, professors, and scholars to stop by and exchange ideas!
About bioGenous
bioGenous is a leading one-stop provider of organoid technology products and solutions. The company has been deeply involved in basic organoid research and translational applications for many years. Since 2016, bioGenous has gradually established and perfected a full range of upstream organoid technologies, covering multiple tissue and tumor organoid culture, development and disease modeling, genetic manipulation, efficacy and toxicity evaluation, animal transplantation, multiomics atlas of organoids, organoid precision medicine, automated operations, and more. In 2020, to meet the explosive global demand for CDMO services in organoid technology, bioGenous built a 2,000-square-meter production facility that complies with international GMP standards for biopharmaceuticals. The company is committed to creating a standardized and modular CDMO service platform for basic biological and medical research, drug development, and precision medicine, leading a revolution in biomedical models and advancing human health.









